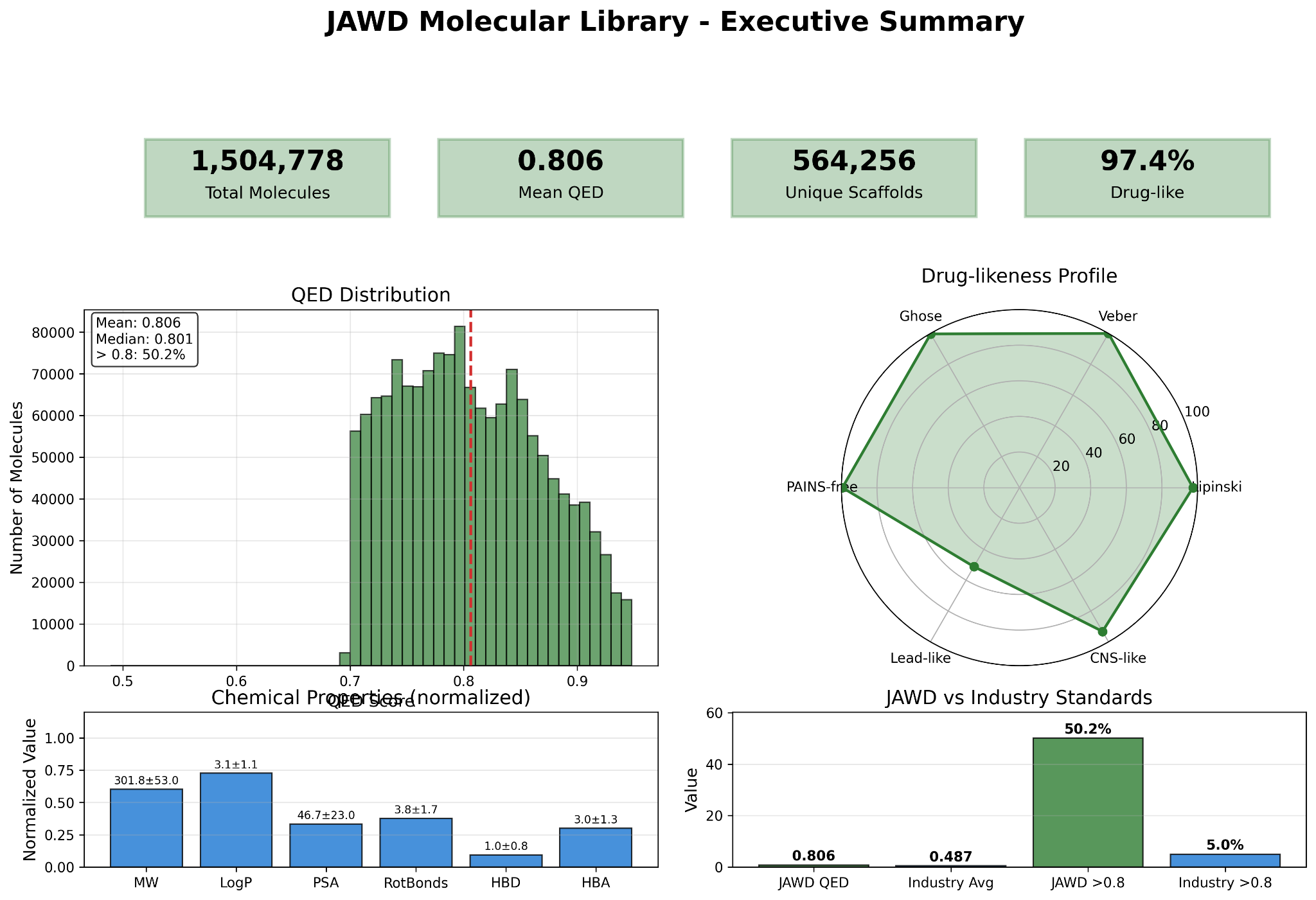
Library overview
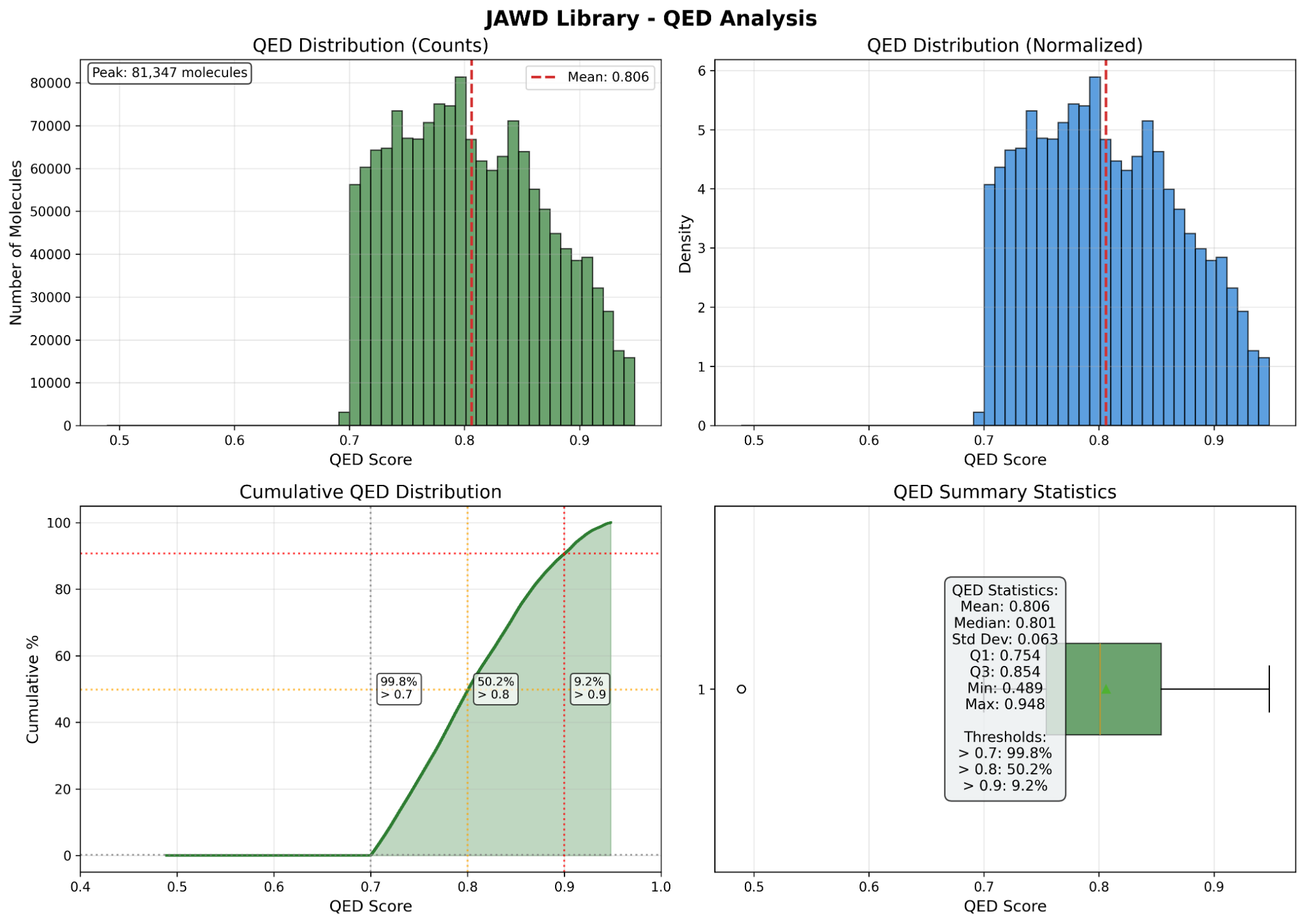
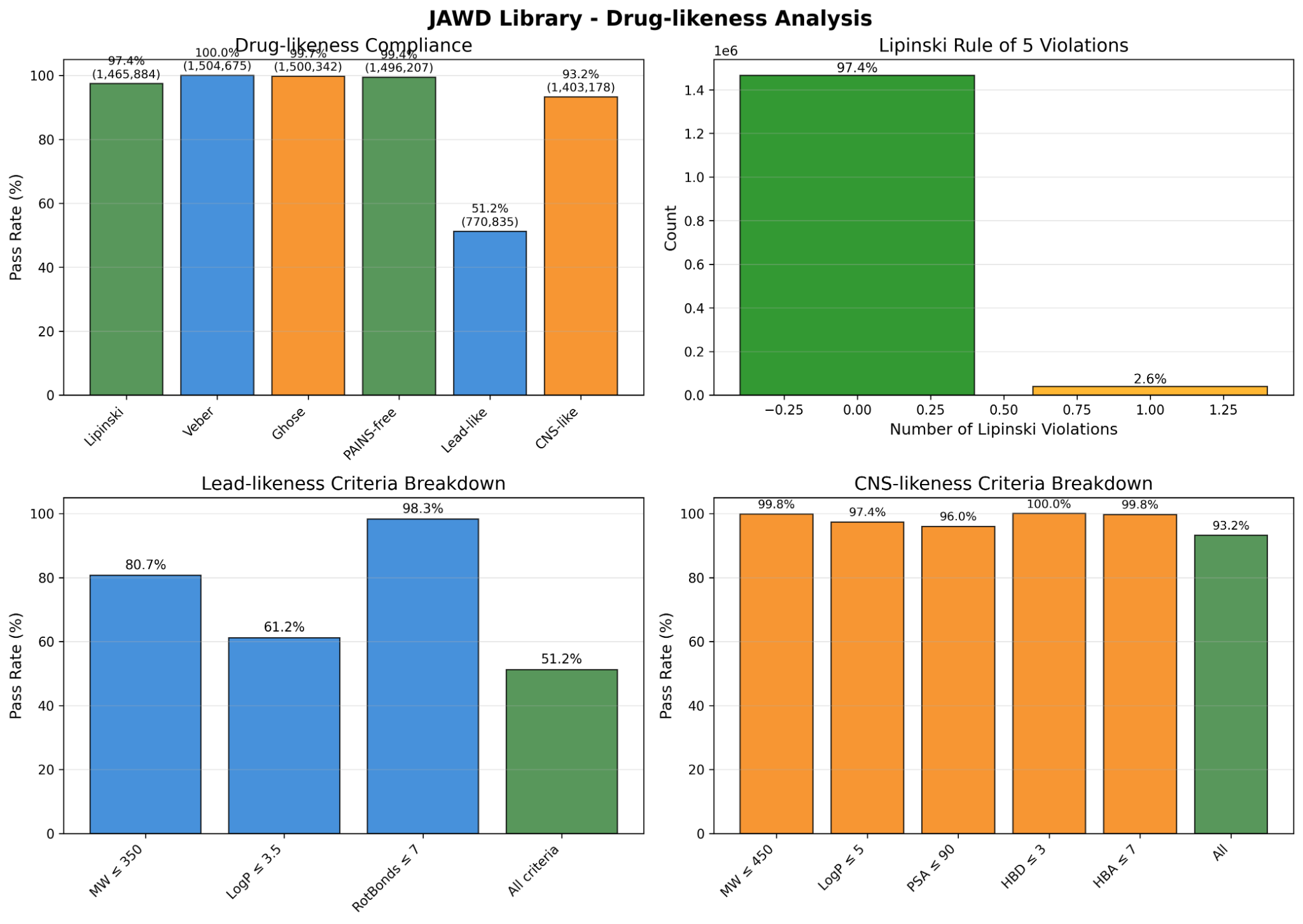
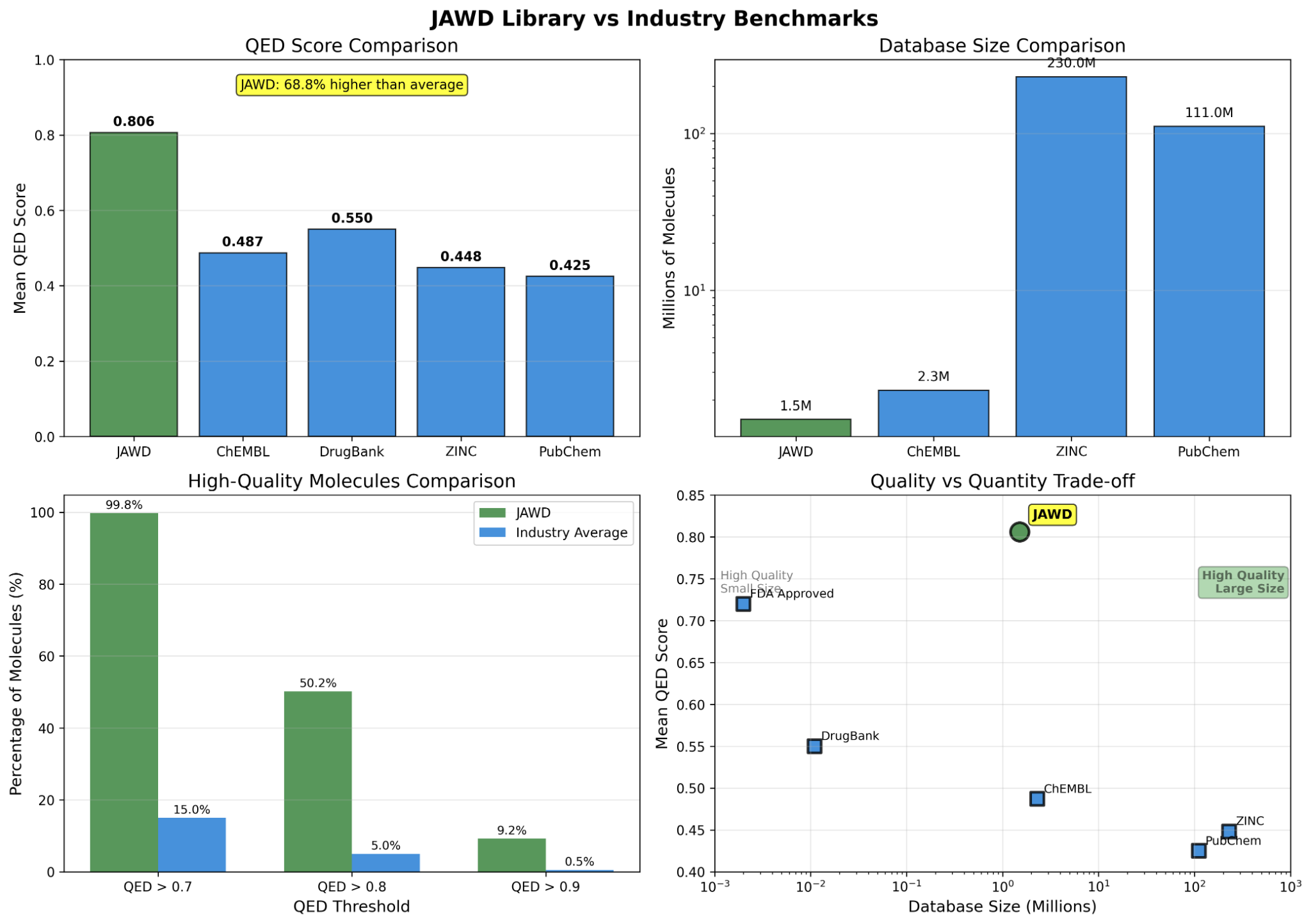
- Total size: ~1.5M molecules; high drug-likeness (QED-focused), PAINS filtering, strong Lipinski/Veber/Ghose compliance.
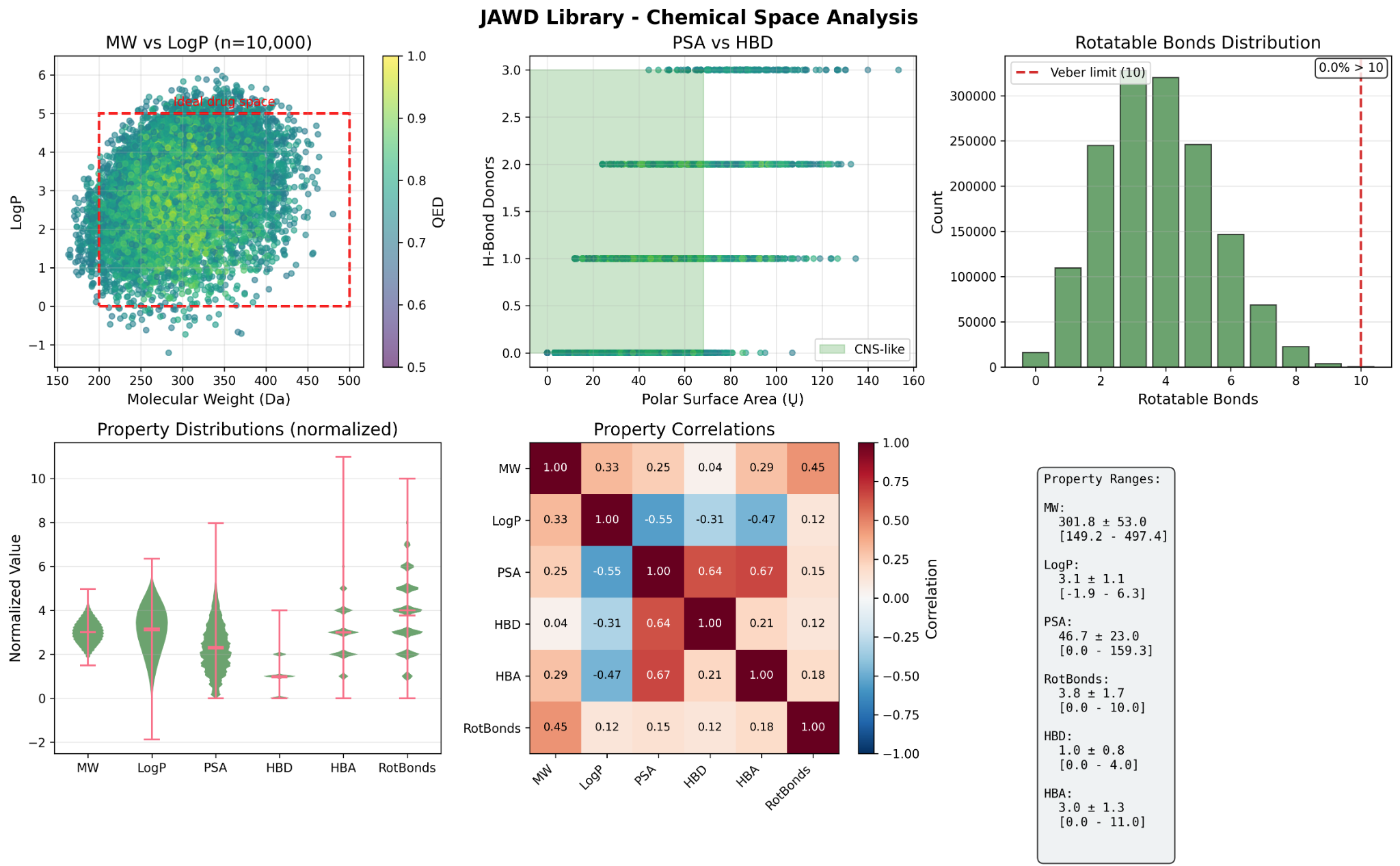
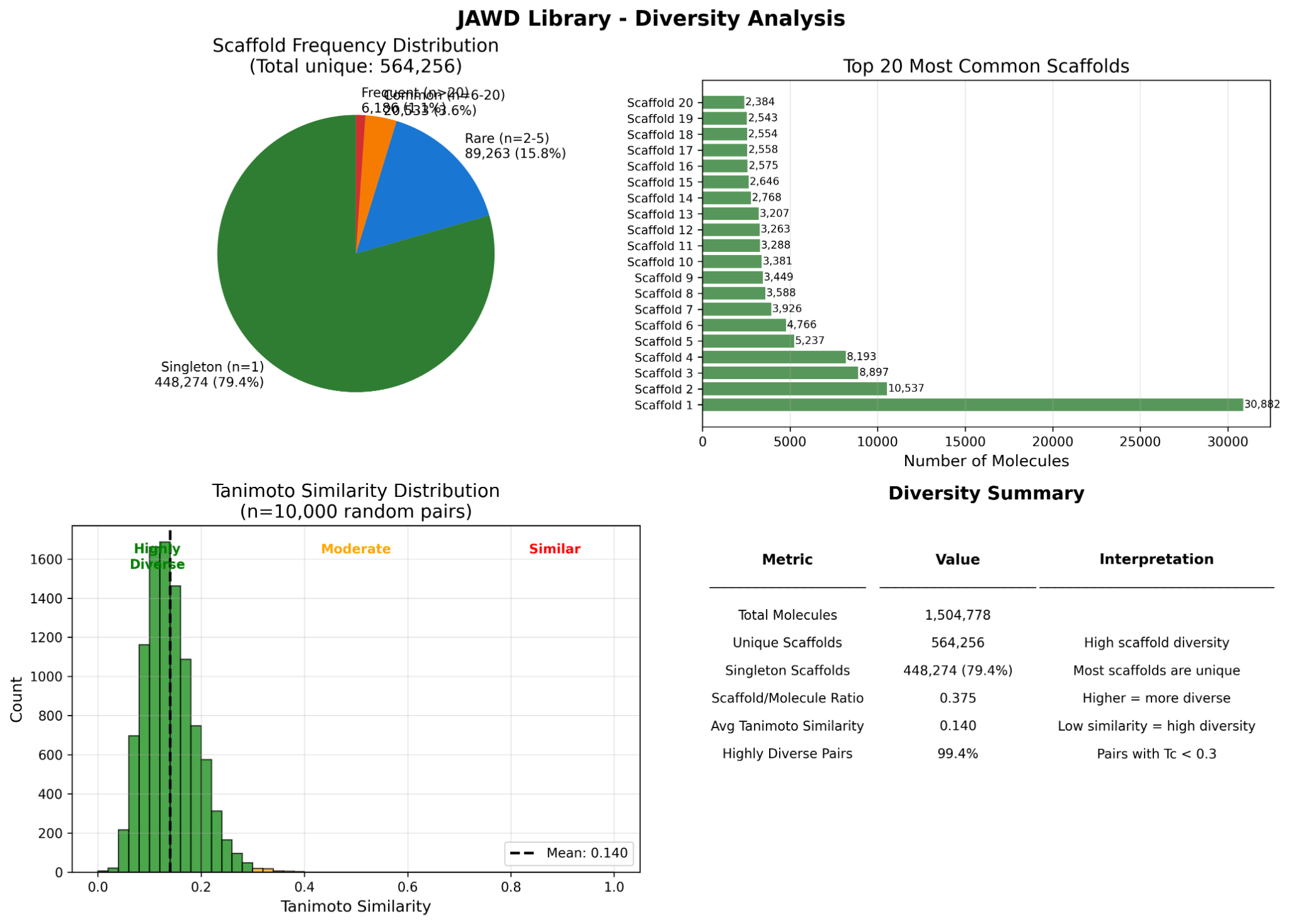
- Broad chemical-space coverage with many unique and singleton scaffolds; low average Tanimoto similarity.
- Deliverables: full dataset, property-selected sub-libraries (CNS-like, lead-like, logP/PSA windows, rotatable bonds, ring counts, scaffold filters, QED cutoffs), or entirely new on-demand AI generations.
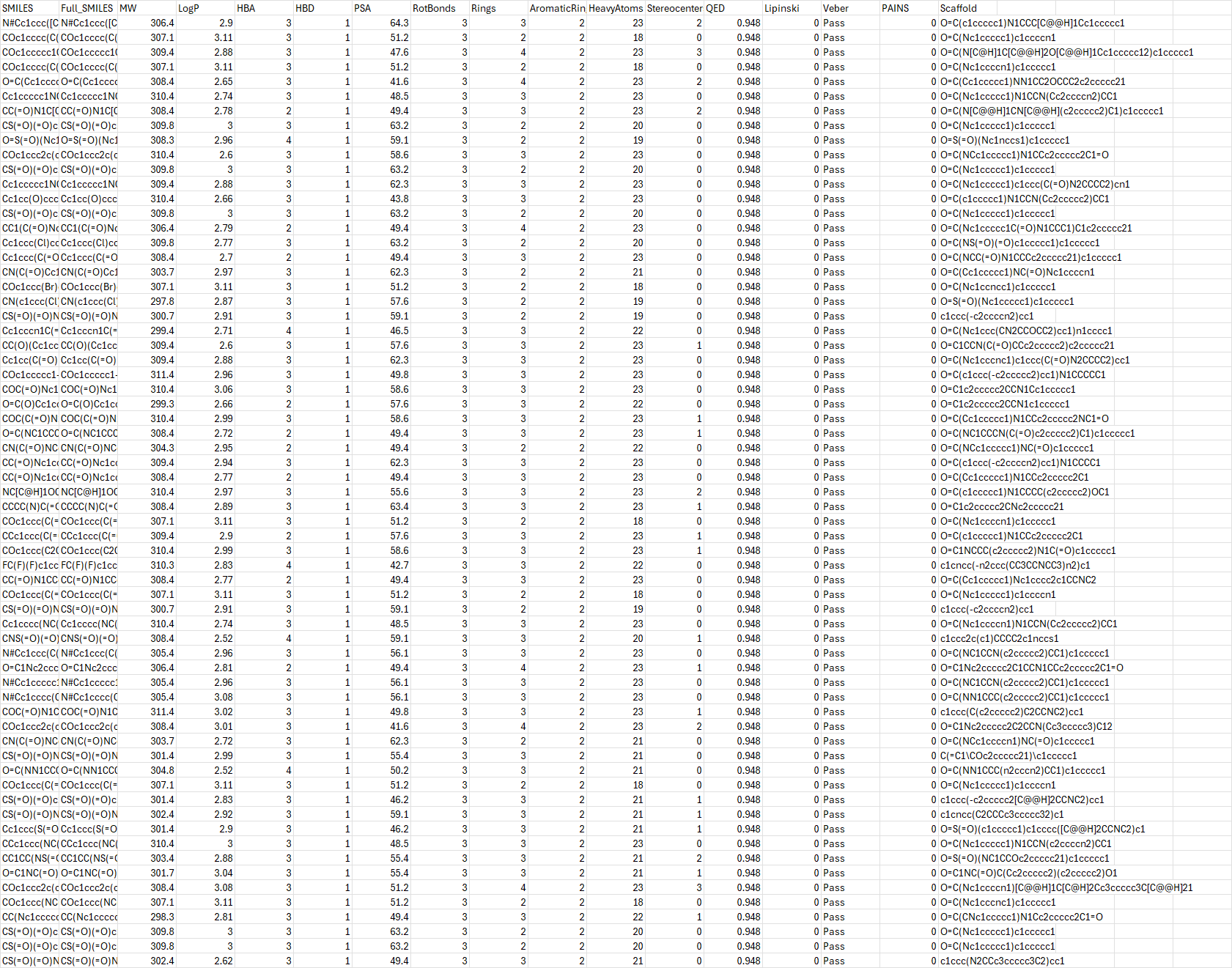
Free samples — 300 molecules (on-page)
Use search or QED filter. Copy/paste directly or use the CSV link above.
CSV (embedded) — click to view
SMILES-only — click to view
Data gallery
Click any image to enlarge.
License & disclaimer
- Research / evaluation only. No clinical, diagnostic, or manufacturing use.
- No redistribution of the sample without written permission.
- AI-generated content. Molecules may be hypothetical and not synthesized; properties are computed in silico.
- No warranties; no liability. Provided “as is” without any express or implied warranty.
- Compliance. You are responsible for any regulatory, IP, ethical, and safety due diligence.
Contact
Email: jawdas@jawdas.com
Email us
We sign NDAs. Enterprise and research partnerships welcome.